-
-
CL Solutions
Cathodoluminescence solutions that reveal fundamental properties of matter
-
Cryo Solutions
Integrated and automated solutions to u nlock the power of cryo - ET workflow -
FAST IMAGING
Fast EM solutions for reliable and high throughput electron microscopy
-
CLEM Solutions
Integrated correlative microscopy solutions that combine the power of fluorescence and electron microscopy
-
CL Solutions
- Contact
- All resources
-
CL Solutions
Cathodoluminescence solutions that reveal fundamental properties of matter
-
Cryo Solutions
Integrated and automated solutions to u nlock the power of cryo - ET workflow -
FAST IMAGING
Fast EM solutions for reliable and high throughput electron microscopy
-
CLEM Solutions
Integrated correlative microscopy solutions that combine the power of fluorescence and electron microscopy
Cryo Solutions
Cryo-electron microscopy: frequently asked questions
Answering the most common questions about cryogenic electron microscopy and cryogenic electron tomography.
Topics
Cryo-electron tomography
-
Why is CLEM useful/necessary in cryo-ET workflow?
Identifying the ROI to mill in the right location is a crucial step, since being off-target for this process could result in milling away your structure of interest. To overcome this, cryogenic fluorescence light microscopy (cryo-FLM) is often used. In this technique biomolecules of interest are fluorescently labelled and the sample is first imaged with cryo-FLM to identify the ROI. The fluorescent image is often correlated with the SEM or FIB image (CLEM) to guide the milling
-
What is the current lamella preparation workflow in cryo-ET?
In cryo-ET a sample is first flash frozen, then thinned to the appropriate thickness and finally a series of images are captured with a transmission electron microscope. To thin the sample, a focused ion beam with a scanning electron microscope, a FIB/SEM, has become the gold standard. The FIB is used to sputter away material and create a thin section called a lamella. To make sure your structure of interest is present in this lamella, cryogenic fluorescent light microscopy is often first used to locate your region of interest.
-
How can cryo-ET be used to study membrane-trafficking?
The field of membrane trafficking focusses on the network of membrane-bound compartments and vesicles that transports molecules to different places within the cell. Most often, live-cell fluorescence microscopy is used to capture these fast and dynamic events. However, the superior resolution of EM combined with the 3D information of tomography can give unique insights into the architecture of the complex network of vesicles and tubules that make up the membrane trafficking system.
Moreover, cryo-ET combined with subtomogram averaging allows the study of protein complexes on their specific membrane domains in near-native state. Cryo-ET holds the promise to take the field of membrane trafficking a step further by combining 3D high-resolution near-native morphological detail of intracellular membranes with structural information of membrane-resident protein complexes.
Learn more how can an optimized cryo-ET workflow benefit membrane trafficking research?
Cryo fluorescence microscopy
-
What are the current challenges of cryogenic fluorescence light microscopy?
Conventionally, the fragile sample needs to be transferred from the cryo-FLM to the cryo-FIB/SEM and then to the cryo-TEM, all while maintaining a temperature below -160°C. To complicate things further, the sample is often transferred back to the cryo-FLM after FIB milling to confirm that the ROI is still present in the lamella. These transfer steps make the workflow an exceedingly challenging task that translates into a low sample preparation success rate of approximately 20%.
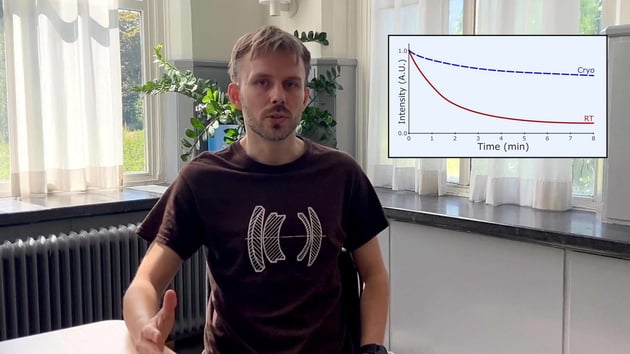
Another challenge is related to ice contamination during cryo-FLM imaging at atmospheric pressure. Although the use of nitrogen vapour in a cryo sample stage of a cryo-FLM is effective in keeping the samples vitrified, it does not completely prevent ice contamination due to residual moisture in the air.
At Delmic we have designed several products that integrate FLM into a cryo-FIB/SEM system. These designs abolish the need for a separate cryo-FLM system and most importantly, reduce sample handling. The cryo-FLM imaging also takes place within the high vacuum environment of the SEM chamber. Using an integrated FLM in the FIB/SEM means that ROI identification by FLM, SEM imaging, FIB milling and ROI confirmation by FLM can all be done in the same system without the need to handle the sample, thus increasing the success rate of the cryo-ET workflow considerably.
Learn more about the limitations and possibilities of cryo-FLM -
Is fluorescence imaging different under cryogenic conditions?
The spatial resolution of cryo fluorescence microscopy (cryo-FLM) is more limited compared to the resolution that fluorescence microscopy (FLM) at ambient temperatures can achieve. This is because cryogenic conditions inhibit the use of immersion objectives. In light microscopy, the high numerical aperture (NA) of immersion objectives determines the resolution achievable, as calculated by Abbe's diffraction limit formula. With an oil immersion objective (NA 1.4 or higher) it is possible to achieve the lateral resolution of around 200 nm1. In contrast, the air objectives that are used in cryo fluorescence microscopy are limited to NA<1 and are therefore only able to reach resolutions of 400 to 500 nm.
In addition to the limitation in NA, cryogenic conditions change many aspects of the fluorophores. This leads to some benefits as well as some challenges for cryo-FLM.
A benefit is that some fluorophores emit more photons at cryogenic temperatures, making each fluorophore brighter. However, this upside is cancelled out by the fact that about 80% of fluorophores are excited into a so-called triplet state, from where they do not emit any fluorescence. This means that only 1 in 5 fluorophores is actually contributing to the signal.
Another change is that the half-life of fluorophores is increased dramatically. This means one can use longer exposure times to get more signal without bleaching the fluorophores. However, this also means that molecules in the cell that contribute to autofluorescence have a longer half-life as well. This translates into very high background autofluorescence in certain cell types.
The last change that should be taken into account is that the excitation and emission spectra of the fluorophores can change at cryogenic temperatures. Being aware of these changes and taking them into account can save you a lot of headaches later down the line. At Delmic we optimize our integrated fluorescence solutions for work under cryogenic conditions.
Reference:
1 Abbe, E. Beiträge zur Theorie des Mikroskops und der mikroskopischen Wahrnehmung. Archiv für Mikroskopische Anatomie 9, 413–418 (1873)
Watch the video of our applications specialist talking about cryo-FLM
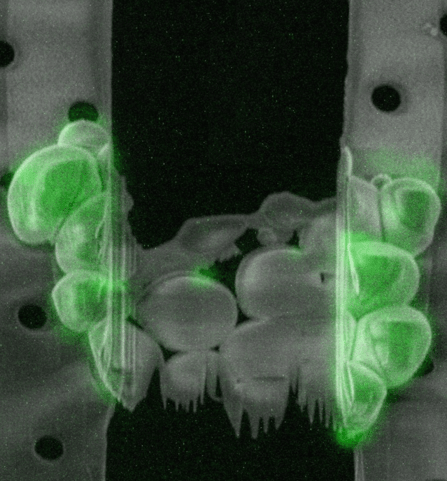
Sample preparation
-
How can cryo-FIB milling be used for thicker samples?
The maximum resolution of a TEM scales down with the increased thickness of the sample. Therefore, to obtain a high resolution tomogram, samples should ideally be between 100 and 300 nm thick. A vitrified sample can be sectioned using cryo-ultramicrotomy: cutting the sample with a diamond knife under cryogenic conditions. This technique, however, has a number of drawbacks, the sections tend to have physical defects, get compressed and wrinkled, making them difficult to image.
The most successful technique to prepare a thin section within the sample, a so-called lamella, is milling using a focussed ion beam (FIB). With this technique, a focussed beam of gallium ions at a high current is used to sputter away the material around the region of interest (ROI) to create a lamella. This process is performed in a scanning electron microscope (SEM) fitted with a FIB (FIB/SEM) that is equipped with a cryo-stage to keep the sample under cryogenic temperatures.
-
How can a ROI be identified in the sample for lamella creation?
ROI’s can be identified using the SEM, but this method is limited to looking at the surface of a cell. This makes it impossible to look for specific molecules or events in a cell especially if they are quite rare. When a protein of interest is tagged with a fluorophore, the ROI can be easily identified using cryo-FLM. Having a separate cryo-FLM makes correlation difficult and adds sample transfer steps that can lead to sample contamination. Integrated FLM makes finding an ROI based on fluorescence easier and prevents extra handling of the sample.
Read more about the sample preparation process -
What are the limitations of the in-situ cryo-FIB lift-out technique?
Cryo-FIB lift-out is a very promising technique that enables the study of in situ structures in multicellular organisms or tissues. There is however still a lot of room for improvement since the production of a single cryo lift-out lamella take up to 10 hours and the success rate is typically smaller than 20%1.
At Delmic we developed METEOR, an integrated FLM solution that could simplify this technique. The integration will reduce the number of transfer steps and reduce surface contamination that can be introduced during transfers. Moreover, having an FLM inside the FIB/SEM chamber allows FLM imaging of the extracted volume to guide the final FIB milling step.
Reference:
1 Schaffer, M. et al. A cryo-FIB lift-out technique enables molecular-resolution cryo-ET within native Caenorhabditis elegans tissue. Nature Methods 16, 757–762 (2019).
Learn more about using integrated FLM to simplify the cryo-FIB lift-out technique

Didn't find an answer?
Fill in the form with your question, and our experts will get back to you soon!
