-
Life Sciences
.png)
Our solutions
-
Materials Analysis
.png)
Our solutions
Techniques
Applications
- Why Delmic?
-
Insights
.png)
Insights
-
Company
.png)
Company
Life Sciences
METEOR and CERES Ice Shield to help shed light on the molecular machinery in organelle membrane and cytoskeletal protein complexes

Prof. Dr. Friedrich Förster is the head of the in situ Structural Biology Lab, part of the Structural Biochemistry group at Utrecht University. His research group is broadly interested in understanding biological assemblies under near-native conditions within the cell.
The focus of Prof. Dr. Förster's group specifically lies in studying protein translation and translocation across the mitochondrial and endoplasmic reticulum membranes, the cell’s responses to stresses caused by the accumulation of unfolded proteins, as well as a relatively new area of cytoskeletal research involving the selection of microtubules by specific kinesin motors. These processes all involve the interplay of diverse regulatory protein networks that are only remotely understood. Since they are extremely challenging to reconstitute biochemically from purified components, analyses in and ex vivo are pursued. Prof. Dr. Förster indicated that his interests do not only lie in understanding what the necessary components are and how they assemble in 3D, but also in the mechanistic details of how they perform their various tasks. He sees cryo-electron microscopy (cryo-EM) as an excellent method to pursue these aims.
Due to the transient nature of many protein interactions, it is often not feasible for Prof. Dr. Förster and his group to isolate the protein complexes and purify them in any significant quantities. They, therefore, relied on very mild cellular disruptions and quick purifications to capture these short-lived interactions and low abundance complexes. In some cases, they were also forced to cross-link the complexes to make the low-affinity interactions amenable to the concentrations suitable for cryo-EM with the caveat that these crosslinks could also introduce some artifacts or limit the resolution. The biggest bottleneck was generally to obtain enough of the pure complex of interest in a functional state. Working in the unperturbed cell would entirely circumvent the restrictions of biochemical enrichment and purification.
Before CERES Ice Shield, Prof. Dr. Förster’s research group faced a major limitation in the preparation of thin lamellae using their dual-beam system, namely the deposition of residual water vapor in the chamber that continuously deposits on the lamella surfaces, adding to their overall thickness. This strongly decreases the contrast in the transmission electron microscopy images and ultimately limits the information and resolution they obtain from the sample. With Delmic CERES Ice Shield and automatic liquid nitrogen re-filler, the in-chamber ice contamination rate became significantly lower, below what can be reliably measured with the dual-beam system’s optics, thus, giving Prof. Dr. Förster and his team opportunities to obtain lamellae of better quality.
Prof. Dr. Förster also selected Delmic’s integrated fluorescence light microscope, METEOR, over other light microscopy solutions since it allowed him to inspect the sample both during and after focused ion beam thinning to ensure that the target of interest stayed within the final lamella.
We believe this is the best solution given the axial resolution of conventional widefield microscopy and even confocal. Furthermore, with an integrated system, the fixed transformations between imaging modalities reduce the time cost of closely monitoring the fluorescence during the thinning process.
By using the combination of CERES Ice Shield and METEOR upgrades, Prof. Dr. Förster believed it would give his research group the best chance of realistically pivoting many research lines and structural questions to the full cellular context. Previously, they were worried about the compromises on throughput and statistical power this would entail, and if the extent of these compromises would ultimately undermine the research. However, the time saved from directly correlating fluorescence data with the sample preparation, as well as the increased number of sites available from each grid due to increased available polishing time, convinced them that they could indeed answer some questions directly in cells.
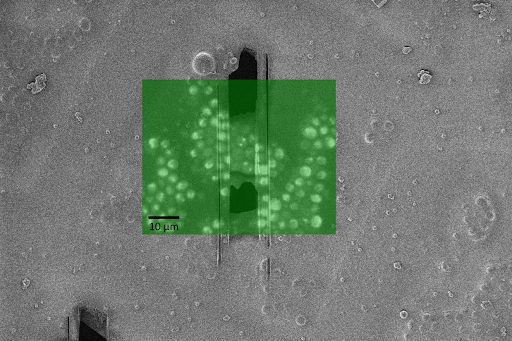
Figure 1: Lamella prepared of budding yeast with GFP-tagged Ire1p and the unfolded protein response induced. Two cells with Ire1p clusters visible in the final lamella.
They also expect that the combined systems will allow them to study the complexes responsible for co-translational import of proteins into the endoplasmic reticulum and mitochondria. Prof. Förster and his team mentioned the vital importance of additional proteins and accessory factors that bind both the ribosome and the nascent polypeptide, yet it is very difficult to describe at molecular resolution.
Translation of mRNA and its downstream events are regulated by a complex network of interactions so intricate and delicate that they are strongly altered when you break the cell – and hence we ultimately need to study the structural basis of their regulation in the cell. For example, we would love to follow in detail how a protein hormone such as insulin is made and whether this process is affected in diabetes. We also hope to understand exactly how different microtubule subsets are recognized by kinesin motors and other associated molecules.
Prof. Dr. Förster acknowledges Delmic’s service and support, especially regarding the METEOR system.
They were sure to arrange a feedback session a few weeks after installation to give us some time to get used to the system and make sure any questions were addressed. They have also been very helpful in suggesting modifications to the calibrations and working procedures to adapt to our specific applications and more challenging samples.




