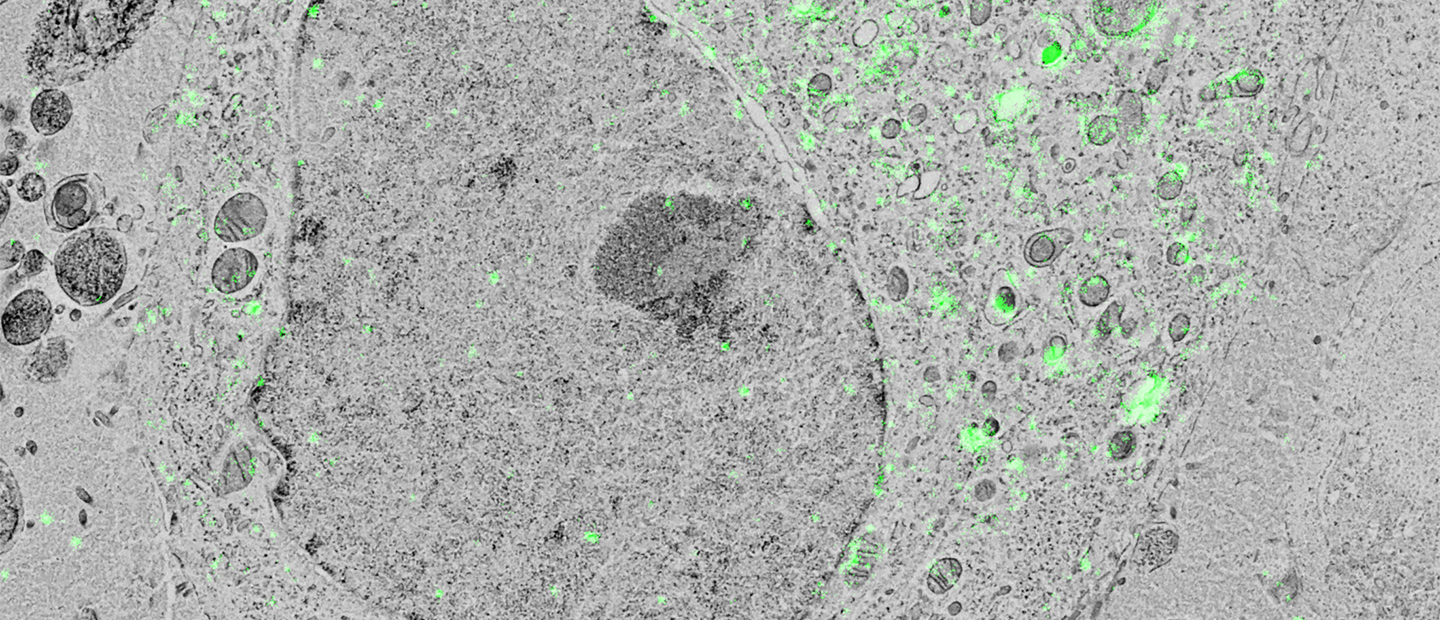
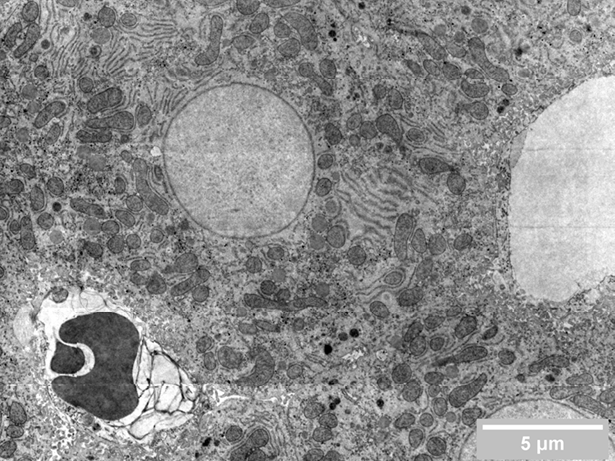
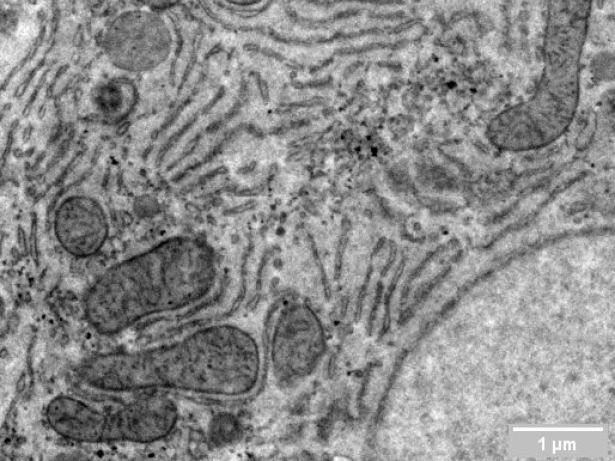
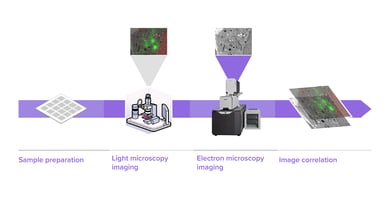
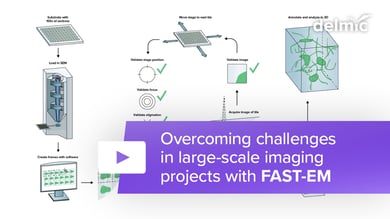
Researchers in cell biology rely on electron microscopy (EM) to answer their research questions since its high resolution is indispensable to visualize the nanoscale details that define individual cells. To analyze various aspects of the complex organization of cells, there is increasing demand to study the same sample at different length scales in biology. Correlative light and electron microscopy (CLEM) is a powerful technique that is used for this purpose. CLEM combines fluorescence microscopy (FM) with high-resolution electron microscopy (EM) to provide information about the composition and function of the sample. However, in traditional CLEM workflows, EM is often a limiting factor for the scope of data collected: with low throughput and limited field of view, careful selection of ROIs is needed to ensure the feasibility and success of a project. Moreover, the limited throughput of the workflow leads to the lack of context, which makes CLEM more of a qualitative technique rather than quantitative.
.png)